6 Label Tips for Cosmetics and Beauty Products
An unattractive label is not a “good look” for a beauty product. Aesthetics are an important factor when investing in labels for cosmetics and beauty products, but it’s not the only aspect that you need to consider. Here are six tips that you should follow when it’s time to design and order custom labels for your beauty products.
1. Follow the FDA’s Cosmetic Label Guidelines
A label design that doesn’t follow the rules isn’t going to do your products any good. Misbranded cosmetic packaging is subject to regulatory action from the FDA if it’s found to be misleading or lack any of the required information.
What Counts as a “Cosmetic” Product?
The Federal Food, Drug, and Cosmetic Act (FD&C Act) categorizes cosmetics as products applied to the body for aesthetic purposes without altering its structure or functions, excluding soaps that only claim to cleanse.
This includes “products such as skin creams, lotions, perfumes, lipsticks, fingernail polishes, eye and facial make-up preparations, shampoos, permanent waves, hair colors, toothpastes, deodorants, and any material intended for use as a component of a cosmetic product.”
Products that serve both cosmetic and therapeutic purposes, such as fluoride toothpaste or sunscreen, must adhere to the regulatory standards for both cosmetics and drugs, facing more stringent requirements like annual FDA registration and adherence to certain manufacturing practices.
FDA Cosmetics Labeling Guidelines
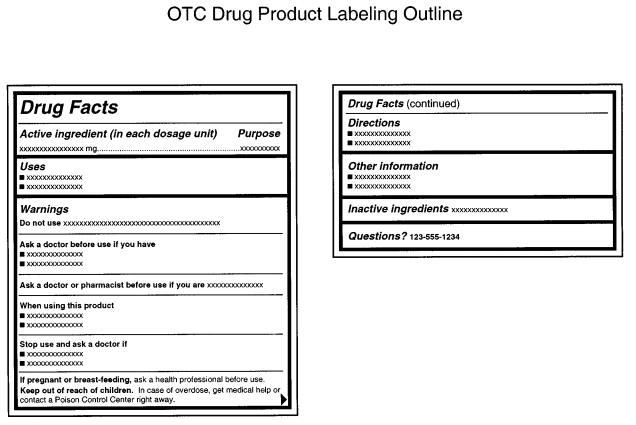
The FDA provides a cosmetic labeling guide that can help you ensure that your product labels follow any regulatory requirements. These include detailed requirements for all accompanying labels and written materials:
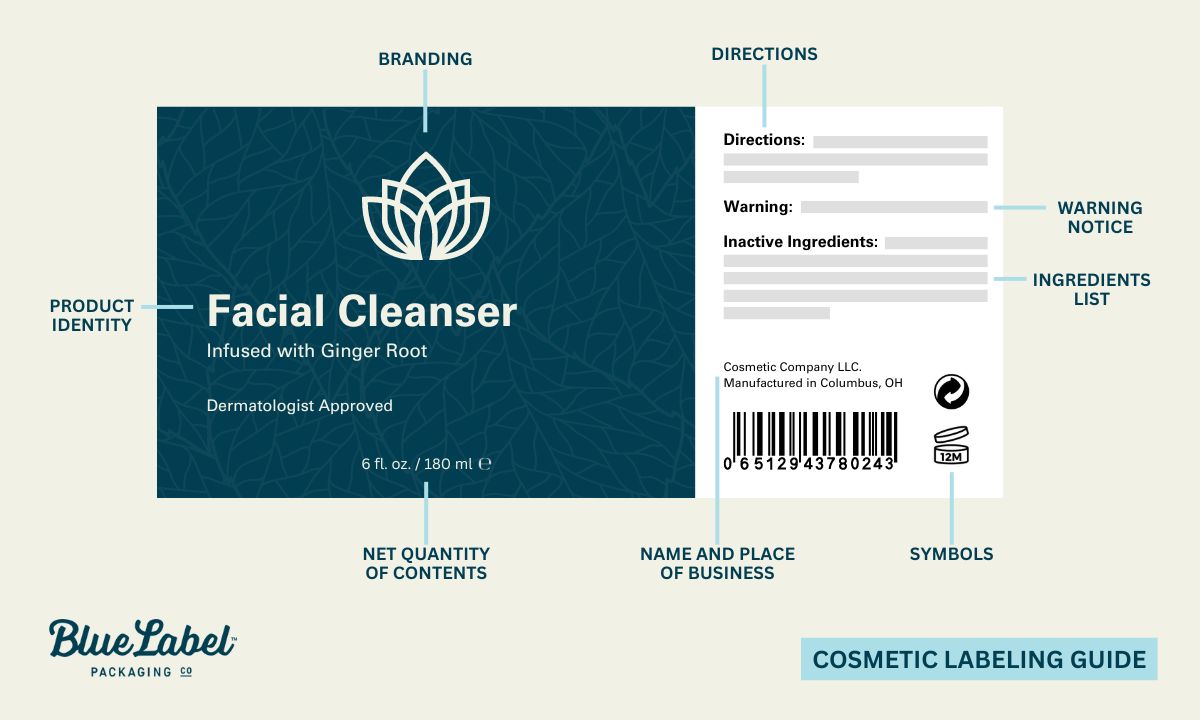
- Label statements required by the FD&C Act must be visible on both inside and outside containers or wrappers.
- Ingredient labeling and net quantity statement is required only on the outer container’s principal display panel, as per 21 CFR 701 and 740 regulations.
- The principal display panel must:
- Display the product name, descriptive nature or use, and accurate net quantity of contents (in weight, measure, numerical count).
- Place the net quantity declaration distinctly at the bottom in a type size regulated according to container size.
- Include sold, semi-solid, or viscous cosmetics in quantities in pound/ounce; liquids in U.S. gallon/quart/pint/fluid ounce. Quantities greater than one pound/pint should also state largest whole units (pounds/ounces or quarts/pints/ounces), optionally including metric measurements.
- The name and place of business of the firm marketing the product should appear on an information panel: includes street address unless listed in current directories but must always include city, state, zip code. If not manufactured by marketer: indicate “Manufactured for …” or “Distributed by …”.
- All imported articles must state on the label the country of origin
Declaration of Ingredients
Cosmetics for retail sale must include an ingredient list, while professional-use products are exempt unless sold to consumers.
- The ingredient declaration should be conspicuous and readable at purchase, appearing on any information panel or affixed tag/tape/card.
- Letter height minimum: 1/16 inch; for packages <12 square inches, min. 1/32 inch.
- Off-package labeling allowed under specific conditions (e.g., cosmetics in compartmented trays without a folding carton).
- Cosmetic ingredients should be listed in descending order of predominance except:
- Color additives and ingredients ≤1% can disregard order.
- Names must follow regulation-established terms; some may be grouped as “and other ingredients.”
- For cosmetics also classified as drugs, drug ingredients labeled first as “active ingredient(s).”
Label Warnings
Cosmetics that could be hazardous if misused must have clear warning labels and directions for safe use, as mandated by regulation 21 CFR 740, especially products like aerosol cosmetics, feminine deodorant sprays, and children’s bubble baths.
While not required by the FD&C Act to test products for safety, manufacturers are strongly encouraged by the FDA to do so; otherwise, they must include a specific warning label indicating the product’s safety has not been determined.
Tamper Resistant Packaging
Liquid oral hygiene and cosmetic vaginal products sold at retail must be in tamper-resistant packaging, featuring a distinctive indicator or barrier that alerts consumers to any tampering.
The package must also clearly display a statement about its tamper-resistant feature, which remains visible even if the feature is compromised, as per Sec. 21 CFR 700.25.

2. Highlight Features and Benefits
The FDA’s required information isn’t the only item that you’ll want to include on your product labels. Highlighting product features and benefits can be a good way to help distinguish yourself from competitors, especially if certain claims can be a deciding factor for your target audience. These types of claims include:
- Organic: Featuring “organic” on labels assures consumers that the product contains naturally sourced ingredients, appealing to those seeking chemical-free beauty options.
- Vegan: Highlighting a product as “vegan” communicates it contains no animal-derived ingredients, catering to ethical and environmentally conscious shoppers.
- Cruelty-Free/Not Tested on Animals: A “cruelty-free” claim signals that the product and its ingredients were not tested on animals, aligning with the values of compassionate consumers.
- Alcohol-Free: Labeling products as “alcohol-free” can attract customers looking for gentle formulations that won’t dry out or irritate sensitive skin types.
- Hypoallergenic: The term “hypoallergenic” suggests a lower risk of allergic reaction, making it desirable for individuals with sensitive skin seeking safe beauty solutions.
If any of these claims are truthful and not misleading, they can be added to your product label.
Another piece of information that’s good to include is an expiration date. U.S. law currently doesn’t have any regulations involving expiration dates, but each company does have a responsibility for the safety of their products. If your products will expire, it’s best to include that date on your label for the good of your customers.
3. Think About Label Shapes and Sizes You’ll Need
Unless you carry one type of product, there’s a fair chance that your labels aren’t going to be a one-size-fits-all solution. Common cosmetic containers include:
- Airless bottles
- Pumps
- Sprayers
- Jars
- Tubes
- Droppers
- Compacts
Each one of these containers can call for labels of different shapes and sizes. This can be a serious issue if you’re banking on using the same exact design for every product.
While you can certainly use the same color scheme, font choices, and other design details for each container, you’ll want to make sure that you adapt your branding and any legally-required information to the various label shapes needed for every applicable container.
4. Consider Special Printing Techniques and Materials
Don’t be afraid to take advantage of special printing techniques for your cosmetics and other beauty products. These value-added services can add both style and function to your product labels.
Durable Finishes
For beauty products and cosmetics that are often exposed to moisture and high humidity, choosing the right finish can ensure the label maintains its integrity. Finishing techniques like lamination, UV varnish, or an ultra gloss finish are commonly used to protect the label from smudging, tearing, and fading.
Double Sided Labels
If you have a clear container, double sided labels are an eye-catching way to make the most of available space. These labels can be read through your container, adding some additional depth to the look of your packaging.
Hot Foil Stamping
Whether you want to make your product look flashy or sophisticated, hot foil stamping can add a dash of style to your labels. Traditional hot foil stamped labels feature gold and silver, but you have many other options that can change your product’s look, such as a matte black stamp or a holographic sheen.
Embossed Labels
Labels aren’t just a visual medium. Label embossing physically raises specific aspects of your label, adding a three-dimensional look and feel to your beauty product labels.
Waterproof Labels
Your labels shouldn’t fall off until long after your consumers are done with your products. If there’s a chance that your products will come into regular contact with water, you should consider investing in waterproof labels.
Hang Tags
Ever wish you had some extra label space? Custom hang tags give you some extra real estate for brand information, promote special deals, or include any other key details that just won’t fit on your labels.
5. Choose the Best Adhesive for Your Product Label
Having an elegant cosmetic label is only beneficial if it remains attached to the surface of the product. Often custom beauty products like face wash, lotion, perfume, body scrub, and shampoo are used around showers and sinks, leaving them exposed to water, wide temperature ranges, and high-humidity environments.
Without proper adhesive, the integrity of the product label can become compromised making it harder to read or causing it to fall off completely. Below are a few factors to consider if your label is going to last in a high-moisture environment.
Adhesive Performance
As beauty and cosmetic labels are exposed to water, alcohol, plasticizers, and other harsh substances, they must maintain their adherence. Selecting a high-quality adhesive will help to not only prevent your custom label from falling off, but prevent it from losing its appearance as well.
Types of High-Quality Adhesives
Typically, for products that aren’t exposed to humidity a standard all-temperature adhesive will work perfectly. However, if your product is going to be used frequently in a humid environment your adhesive will need to have a high solvent resistance. This will help your product label maintain its stickiness when exposed to water, alcohol, or different solvents.
Along with a high solvent resistance, having an adhesive with high shear resistance is recommended for most custom beauty products. You will want your label to be able to withstand constant stress without tearing or cracking. This is especially true if your product is something that must be squeezed.
Pairing Your Adhesive with Your Product Packaging
It is important to consider your label’s material and the packaging of your product when selecting an adhesive. Many manufacturers choose soft-touch containers to give the bottle a better velvety feel. However, in order to attain full adhesion, a very aggressive permanent adhesive is needed. Likewise, any custom clear film labels will require ultra-clear permanent adhesives.

6. Design an Irresistible Product Label
Walk down any beauty aisle and you’ll find shelf after shelf with stunning designs. Designing a compelling cosmetic product label means blending aesthetics, functionality, and branding in a way that captivates potential consumers at first glance.
- Use color psychology to your advantage: Incorporate colors that evoke emotions and perceptions related to your product, enhancing the consumer’s connection and desire for it.
- Use unique typography and visual hierarchy: Use clear typography and a structured visual hierarchy to make essential information easily navigable, ensuring consumers can quickly find what they need.
- Set it apart with textured surfaces or unique shapes: Add textured surfaces or unique label shapes to introduce a tactile dimension that stands out on the shelf, inviting physical interaction.
- Tell a story through design: Craft a narrative with your label design to develop a deeper bond between the consumer and your product.
- Adopt minimalistic design approach: Embrace minimalism for a clean, modern aesthetic that highlights key features of your product without overwhelming consumers with information.
- Color match your product: Consider matching your label color with the cosmetic inside for cohesive packaging that visually communicates the product’s look before it’s even opened.
- Try eye-catching patterns: Test out irregular or abstract patterns in designs to give packages an edge while still maintaining a broad appeal.
Find the Right Professionals
It’s not always easy to create a quality label for beauty products. That’s why it’s best to invest in a good designer and printing company to ensure that your product labels are as good as they need to be to attract new customers.
At Blue Label, our digital printing technology gives us the ability to quickly and efficiently print quality labels. Not only can we handle special printing techniques to make your labels stand out, we can also group multiple label versions in the same order to save you money. If you’re still looking for a label designer, our designer directory can help you find a partner to help you bring your vision to life.
Ready to invest in quality cosmetics labels? Contact us today to talk to one of our experts about how we can help you with your label printing project.