4 Common Mistakes for CBD Labels
As the CBD market grows, more products will flood the market. This growth means that your CBD your products need to stand out from the rest of your competitors. That’s where a good CBD label can help. Proper packaging can be the difference from your product being just another item on the shelf or a big success. However, it’s easy to make a few notable label mistakes during the process. Here are four issues you should avoid for your CBD labels.
Some CBD Labels Don’t Include Legally Required Information
What’s tricky for CBD labels is that the federal government isn’t quite clear about the exact guidelines for these types of products. However, it’s a good idea to list out the following details to provide your audience with the right information.
- The amount of active CBD per serving
- A supplement fact panel that includes all ingredients
- Net weight
- The manufacturer or distributor name
- Whether the CBD used is full spectrum, broad spectrum, or an isolate
- A batch or date code
- The suggested product use
Of course, there may be more to your labeling requirements than just that. CBD products may require some other information depending on how you classify the product. For example, a food product needs to follow the the regulations found in the FDA Food Labeling Guide. However, a healthy and beauty product needs to adhere to the FDA’s rules on cosmetic labeling. Once you take note of how your product is classified, you can then apply those labeling guidelines to your product in addition to including general compliance information for CBD.
Certain CBD Labels Deal with Font Issues
It’s hard to get your product’s message across when there’s something wrong with the type on your CBD labels. Text and font issues can prove problematic for any product. For CBD labels, a wrong font can not only muddle the look of your label, it can also land you into some compliance trouble.
When you deal with labeling requirements set by the FDA or some other administration, there are occasions where you need to use a certain font size or style. These rules are in place to ensure that certain details are easily read, so it’s best to abide by them. However, they may not be as simple as following a set font size.
For example, the FDA requires labels to use “a print or type size that is prominent, conspicuous and easy to read” for information panels. Seems simple right? Just wait, there’s more! The labeling guide also states that labels should “use letters that are at least one sixteenth (1/16) inch in height based on the lower case letter ‘o,’” except in the case of “very small food packages as discussed in 21 CFR 101.2(c) & (f).” Finding the exact type rules for your exact product may require some digging, but it’s still preferable to having a federal organization confiscate your products and fine you for improper labeling.
There are also occasions when the type used isn’t necessarily a legal issue, but does pose some design issues. This problem is especially true for CBD products sold in small containers that only provide a few inches of labeling space. At this point, you’ll need to balance both compliance and design to find a typography compromise [link to new typography tips post when it’s live] that showcases your brand without coming off as busy or boring. Take the label from Limitless CBD pictured below as an example. Despite not having much space to work with, Limitless’ design establishes a clear identity with legible text, all while meeting regulations.

Other CBD Labels Get Caught Making Health Claims
There may be a lot of studies that suggest that CBD has significant health benefits, but there isn’t enough existing information to convince the FDA of them. The amount of documented information required makes it tricky to legally make a health claim on a label for any product. For CBD products, it’s nearly impossible.
As of now, the FDA’s stance is that “there are many unanswered questions about the science, safety, and quality of products containing CBD.” This position means that federal law does not recognize CBD as a dietary supplement or as a substance that can help prevent, diagnose, treat, or cure serious diseases. The only exception to this is a single prescription CBD product that has been approved to treat rare forms of epilepsy.
Aside from that very specific breakthrough, the FDA will crack down on CBD labels that make health claims about cancer, diabetes, Alzheimer’s, or any other conditions. As such, it’s best to avoid these claims altogether on your packaging if you want to avoid potential legal intervention.
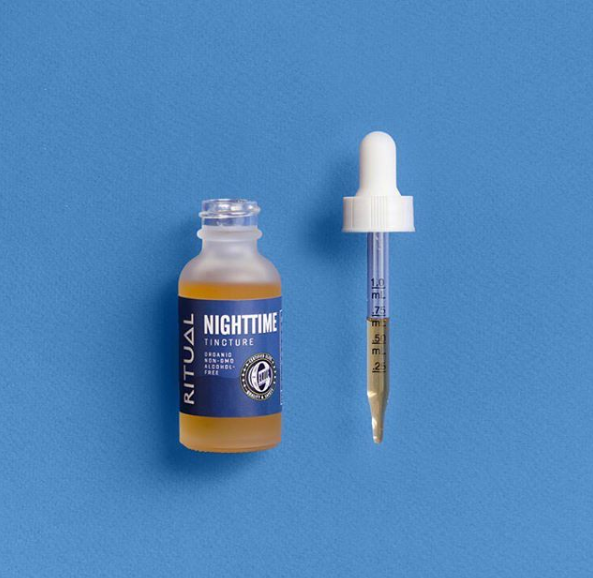
However, the potential dangers of health claims doesn’t mean that you can’t highlight other beneficial features. For example, it’s completely fine to state that your product is organic, GMO free, or something similar as long as your product meets the legal guidelines for such terms, such as the label for Ritual’s Nighttime Tincture pictured below.
They Showcase CBD Too Much
As we mentioned eariler, the CBD market is booming at the moment. This has led to scores of new consumers trying to find the appropriate CBD product for them. That increase in potential customers is great if your product meets their needs. However, you have to be careful that your consumers see you as more than just another CBD product.
While a big ol’ “CBD” on the front of your product may attract the random customer, there’s something to be said for subtlety. If a consumer only knows you as “that CBD product I use,” what’s to prevent them from seeing you as interchangeable if a bigger, shinier CBD product emerges? Instead, it’s important to focus on designing your CBD label to focus on your brand and developing a relationship with your intended audience. This way you become a name to them, and it’s easier to create brand loyalty if they remember you as “ABC CBD” than “that green bottle that says CBD on it.”
CBD Social is a great example of establishing an identity that goes beyond the use of CBD. The label pictured below places the emphasis on why the product matter to consumers – extreme relief is awful enticing for someone dealing with pain – and uses CBD to supplement that message in an eye-catching design.

Invest in Professional CBD Labels for Your Products
In a fight for CBD supremacy, the products with the best labels have a massive edge. This is why investing in professionally made, custom product labels can help give your product the boost it needs to tell your story and build a loyal customer base.
At Blue Label, we have the digital printing technology and expertise necessary to create stunning CBD labels for your products. We work with you to determine the best materials and printing capabilities required to meet your performance and budgetary needs without sacrificing on style. If you need one, we can even refer you to our designer directory to find a professional who can balance the creative and legal aspects of your label design.
In a booming market, your CBD products deserve to look as good as they possibly can. Contact us today to talk to us about printing custom CBD labels for your products.

